38 label a chemical equation
KOH + H3PO4 = K3PO4 + H2O - Chemical Equation Balancer WebLabel each compound (reactant or product) in the equation with a variable to represent the unknown coefficients. a KOH + b H 3 PO 4 = c K 3 PO 4 + d H 2 O. Create a System of Equations. Create an equation for each element (K, O, H, P) where each term represents the number of atoms of the element in each reactant or product. 3.10: Writing and Balancing Chemical Equations Steps in Balancing a Chemical Equation Identify the most complex substance. Beginning with that substance, choose an element that appears in only one reactant and one product, if possible. Adjust the coefficients to obtain the same number of atoms of this element on both sides. Balance polyatomic ions (if present) as a unit.
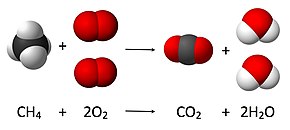
What is the chemical equation for cellular respiration? The overall (unbalanced) chemical equation for cellular respiration is: "C"_6"H"_12"O"_6 + "O"_2 → "CO"_2 + "H"_2"O" + "energy" > The balanced equation is "C"_6"H"_12"O"_6 + "6O"_2 → "6CO"_2 + "6H"_2"O" + "energy" The equation expressed in words would be: "glucose + oxygen → carbon dioxide + water + energy" The equation is formulated by combining the three following processes into one ...
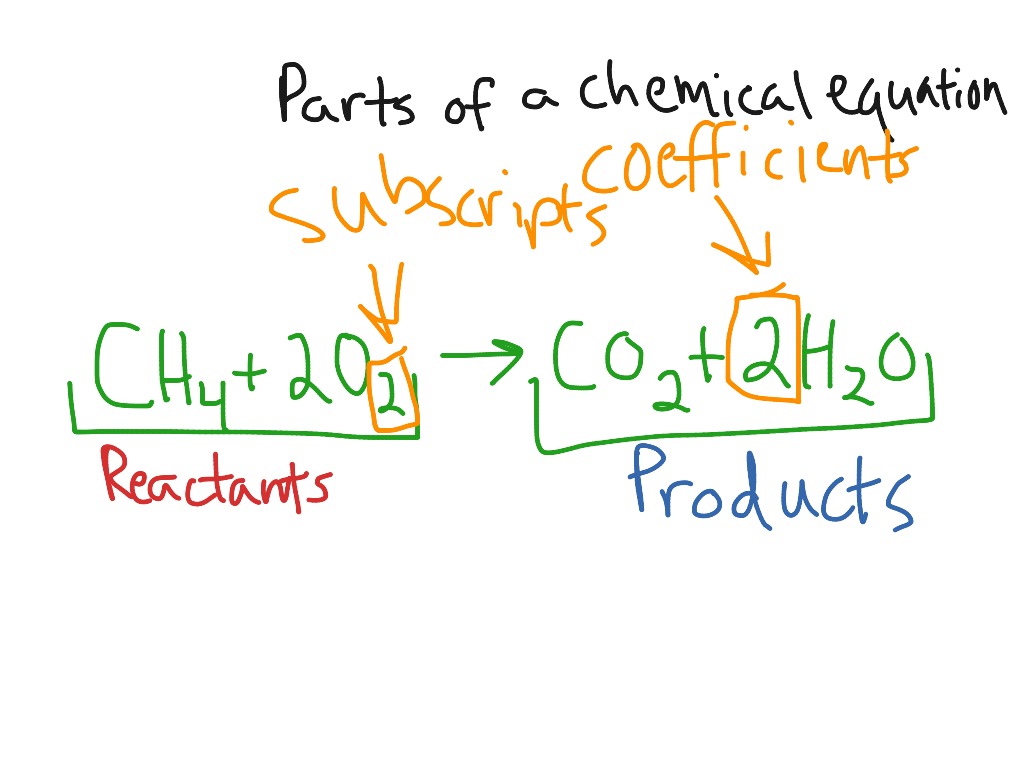
Label a chemical equation
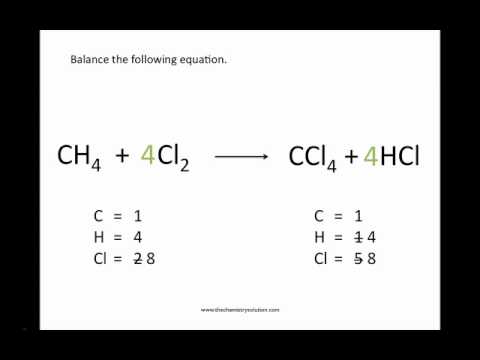
7.3: Chemical Equations - Chemistry LibreTexts Writing Chemical Equations. When sulfur dioxide is added to oxygen, sulfur trioxide is produced. Sulfur dioxide and oxygen, SO 2 + O 2, are reactants and sulfur trioxide, SO 3, is the product. 2SO 2(g) + O 2(g) ⏟ Reactants → 2SO 3(g) ⏟ Products. In chemical reactions, the reactants are found before the symbol " → " and the products are ... Factor-Label Method in Chemistry: Definition, Examples & Practice ... The Factor-Label Method. Believe it or not, one simple method can be used to accomplish many of the basic calculations in chemistry. The method does not involve years of calculus courses or other ... KClO3 = KCl + O2 - Chemical Equation Balancer WebLabel each compound (reactant or product) in the equation with a variable to represent the unknown coefficients. a KClO 3 = b KCl + c O 2. Create a System of Equations. Create an equation for each element (K, Cl, O) where each term represents the number of atoms of the element in each reactant or product. K: 1 a = 1 b + 0c Cl: 1 a = 1 b + 0c O ...
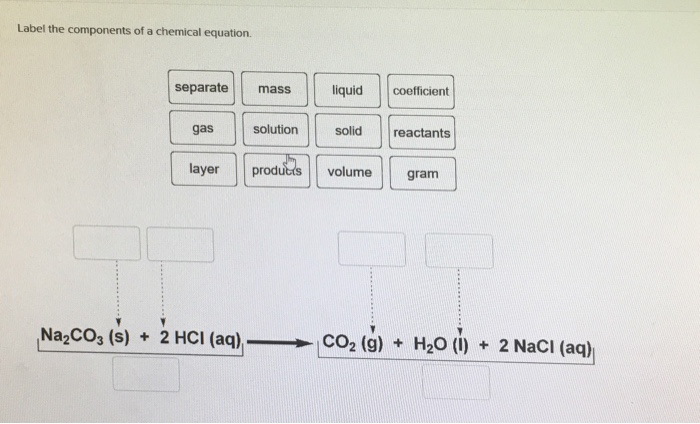
Label a chemical equation. Solved Label the components of a chemical equation. Label | Chegg.com Label the components of a chemical equation. This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer Show transcribed image text Expert Answer Transcribed image text: Label the components of a chemical equation. Label the components of a chemical equation. How do you label a chemical equations? - Answers How do you label a chemical equations? Wiki User ∙ 2013-01-28 13:17:07 Study now See answer (1) Best Answer Copy N2 + h2 =nh3 Wiki User ∙ 2013-01-28 13:17:07 This answer is: Study guides... Solved Label the components of a chemical equation. separate | Chegg.com Science; Chemistry; Chemistry questions and answers; Label the components of a chemical equation. separate mass liquid coefficient gas solution solid reactants layer prod uiets volume gram Na2co, (s) + 2HCl (aq),- ?.co2 (g) + H2O (l) Co2 (g) + H20 ) + 2 Naci (aq) What Are Chemical Equations? - ThoughtCo This can be seen in the following equation: 2 H 2 (g) + O 2 (g) → 2 H 2 O (l) Hydrogen and oxygen are indicated by (g), which means they are gases. Water is marked (l), which means it is a liquid. Another symbol you may see is (aq), which means the chemical species is in water — or an aqueous solution.
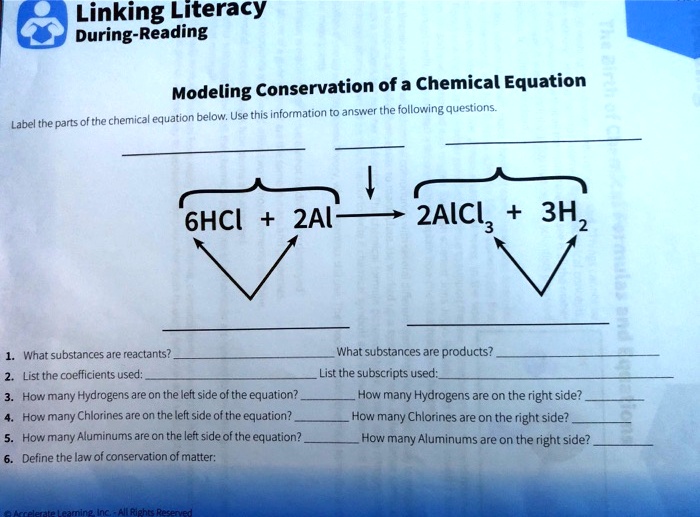
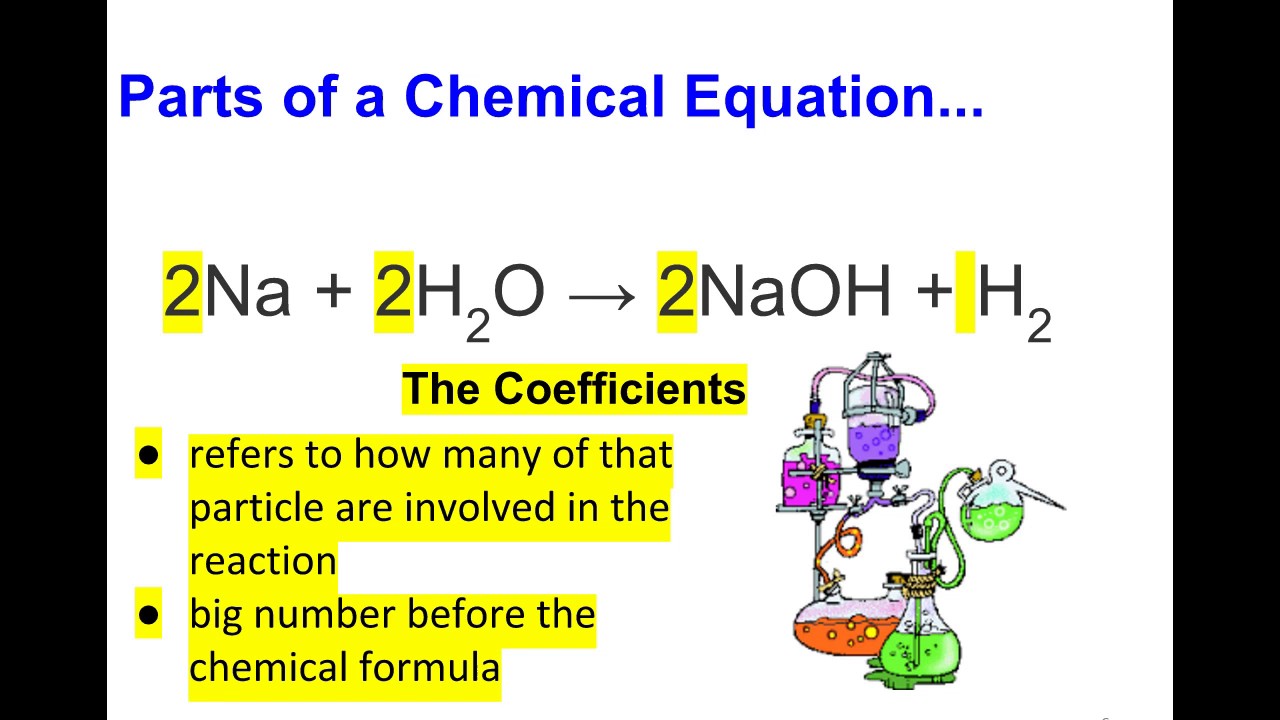
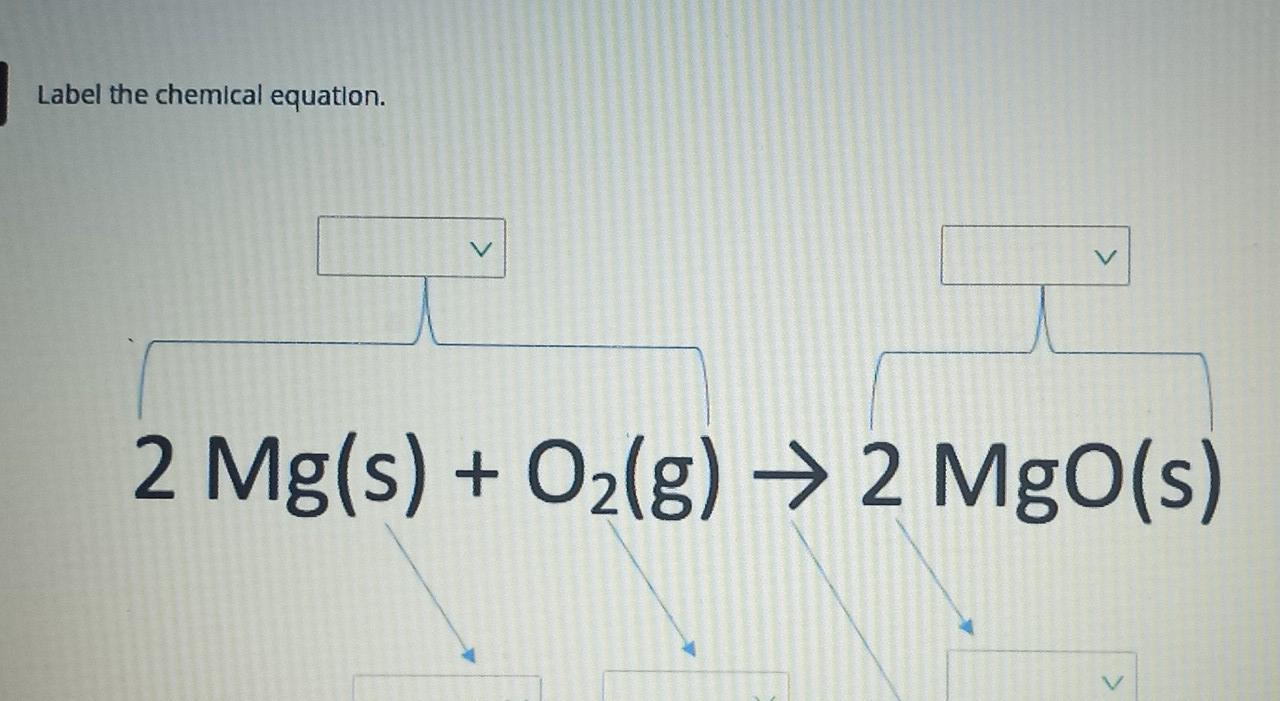
NaOH + H3PO4 = Na3PO4 + H2O - Chemical Equation Balancer WebLabel each compound (reactant or product) in the equation with a variable to represent the unknown coefficients. a NaOH + b H 3 PO 4 = c Na 3 PO 4 + d H 2 O. Create a System of Equations. Create an equation for each element (Na, O, H, P) where each term represents the number of atoms of the element in each reactant or product. Chemical Nomenclature and Chemical Formulas - Owlcation A chemical formula indicates the relative number of atoms of each element in a substance. It consists of symbols of elements and subscripts which give the number of atoms of each element. Examples: The formula of water is H2O. There are 2 atoms of Hydrogen and 1 atom of oxygen. The formula of glucose is C6H12O6. 3 Steps for Balancing Chemical Equations - ThoughtCo 3 Steps for Balancing Chemical Equations 1) Write the unbalanced equation. Chemical formulas of reactants are listed on the lefthand side of the equation. Products are listed on the righthand side of the equation. Reactants and products are separated by putting an arrow between them to show the direction of the reaction. Label the chemical equation. 2 Mg(s) - Brainly.com Label the chemical equation. 2 Mg (s) + O2 (g) → 2 MgO (s) 1 See answer Advertisement fichoh Answer: Kindly check Explanation Explanation: Given the chemical equation: 2 Mg (s) + O2 (g) → 2 MgO (s) 2 molecules of Magnesium + Oxygen gas (Reactant) → ( yield) 2 molecules of magnesium oxide (product) 2 in Mg and MgO (coefficients)
U.S. appeals court says CFPB funding is unconstitutional - Protocol Web20.10.2022 · The 5th Circuit Court of Appeals ruling sets up a major legal battle and could create uncertainty for fintechs. NH3 + O2 = NO + H2O - Chemical Equation Balancer WebLabel each compound (reactant or product) in the equation with a variable to represent the unknown coefficients. a NH 3 + b O 2 = c NO + d H 2 O. Create a System of Equations. Create an equation for each element (N, H, O) where each term represents the number of atoms of the element in each reactant or product. Chemical Equation Calculator - Free online Calculator - BYJUS The procedure to use the chemical equation calculator is as follows: Step 1: Enter the chemical equation in the input field Step 2: Now click the button "Balance" to get the balanced equation Step 3: Finally, the balanced equation, structure, equilibrium constant for the given chemical equation will be displayed in a new window Unbanked American households hit record low numbers in 2021 Web25.10.2022 · Those who have a checking or savings account, but also use financial alternatives like check cashing services are considered underbanked. The underbanked represented 14% of U.S. households, or 18. ...
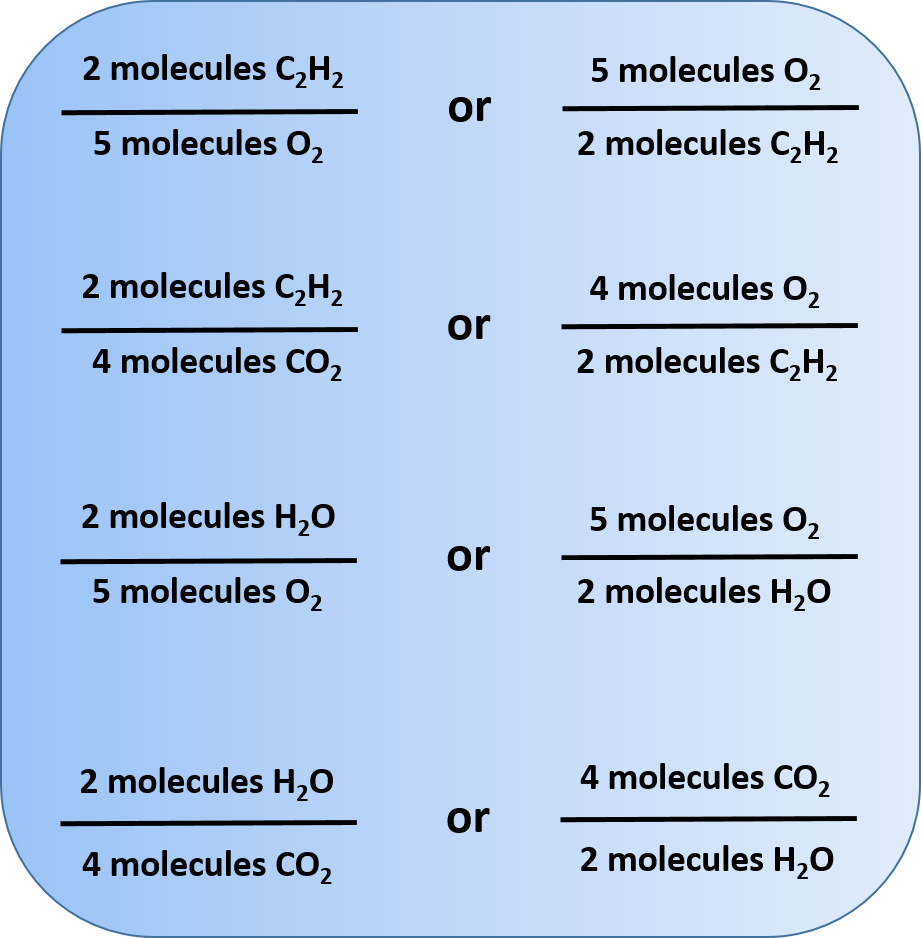
Writing and Balancing Chemical Equations | Chemistry for Majors 4 × 1 = 4. 2 × 2 = 4. 4 = 4, yes. O. 2 × 2 = 4. (1 × 2) + (2 × 1) = 4. 4 = 4, yes. A balanced chemical equation often may be derived from a qualitative description of some chemical reaction by a fairly simple approach known as balancing by inspection. Consider as an example the decomposition of water to yield molecular hydrogen and oxygen.
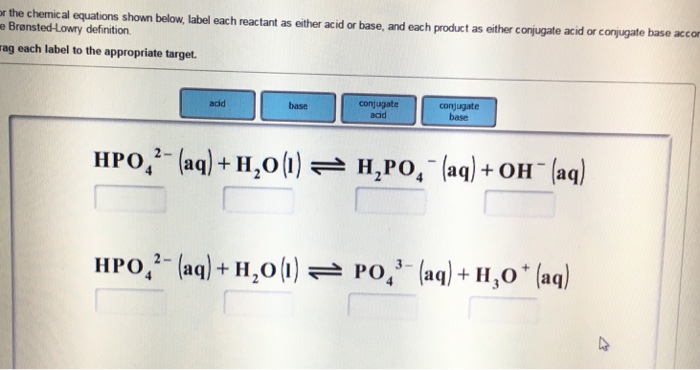
Label each of the descriptions below as theoretical Label each of the descriptions below as theoretical yield, experimental yield, or both. Calculated based on the chemical equation: Measured directly: Determined experimentally: Determined using stoichiometry: DONE 2 See answers Advertisement Tringa0 Explanation:
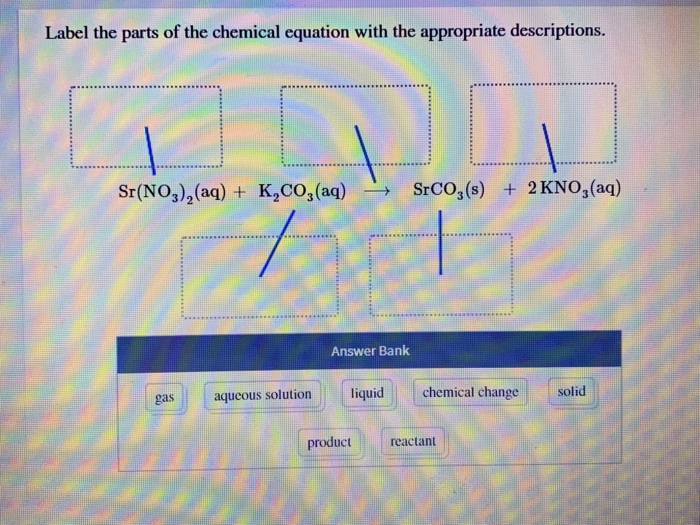
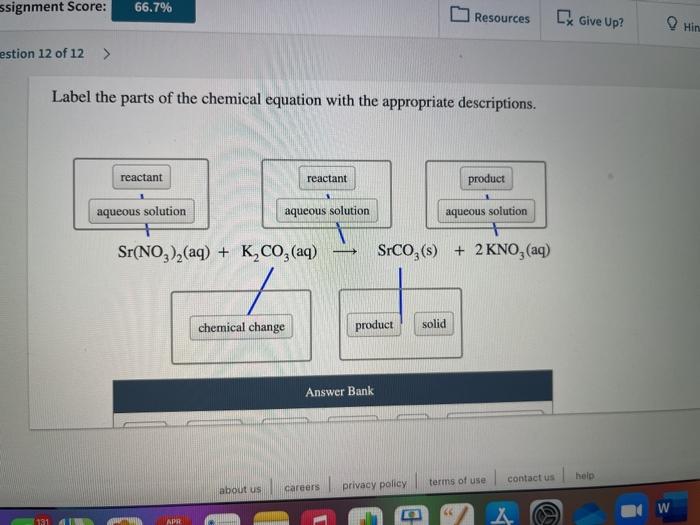
Label the parts of the chemical equation with the appropriate ... - Wyzant Label the parts of the chemical equation with the appropriate descriptions. Label the parts of the chemical equation with the appropriate descriptions. Sr (NO3)2 (aq) + K2CO3 (aq) SrCO3 (s) + 2KNO3 (aq) answer bank-. Product, Liquid, gas, reactant, solid, aqueous solution. Follow • 2.
K + H2O = KOH + H2 - Chemical Equation Balancer WebLabel each compound (reactant or product) in the equation with a variable to represent the unknown coefficients. a K + b H 2 O = c KOH + d H 2. Create a System of Equations. Create an equation for each element (K, H, O) where each term represents the number of atoms of the element in each reactant or product.
Chemical Equation Balancer To balance a chemical equation, enter an equation of a chemical reaction and press the Balance button. The balanced equation will appear above. Use uppercase for the first character in the element and lowercase for the second character. Examples: Fe, Au, Co, Br, C, O, N, F. Ionic charges are not yet supported and will be ignored.
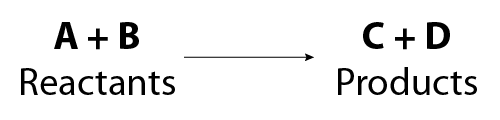
Chemical Equations Flashcards | Quizlet Chemical Equation. symbolic representation of a chemical reaction, will show the same # of each type of atom on each side of the equation. ex. Na + Cl2 -> NaCl. Chemical Formula. symbolic representation of of an element or compound. ex. NaCl (table salt) Chemical Reaction. process in which bonds between atoms are broken and new bonds are formed.
ConferenceSeries LLC LTD | USA | Europe | Asia | Australia WebMeet Inspiring Speakers and Experts at our 3000+ Global Conferenceseries Events with over 1000+ Conferences, 1000+ Symposiums and 1000+ Workshops on Medical, Pharma, Engineering, Science, Technology and Business.. Explore and learn more about Conference Series LLC LTD: World’s leading Event Organizer
Chemical Equations - Definition, Representation, Types A chemical equation is a means of describing a chemical reaction using symbols and equations for the substances involved in the process. The example below demonstrates how to clearly understand the meaning of a chemical equation. Zinc sulphate and hydrogen gas are formed when zinc metal combines with dilute sulphuric acid.
Chemical equation - Wikipedia A chemical equation is the symbolic representation of a chemical reaction in the form of symbols and chemical formulas.The reactant entities are given on the left-hand side and the product entities on the right-hand side with a plus sign between the entities in both the reactants and the products, and an arrow that points towards the products to show the direction of the reaction.
What are Chemical Equations? Detailed Explanation, Examples - BYJUS Chemical equations were first formulated by the French chemist Jean Beguin in the year 1615. Chemical reactions can be represented on paper with the help of chemical equations, an example for which is represented below (for the reaction between hydrogen gas and oxygen gas to form water). 2H 2 + O 2 → 2H 2 O
What are the Parts of a Chemical Equation? | Life Persona The steps followed to write a chemical equation are: - Reagents and reaction products are identified and noted. - The formula or symbols of the reagents are written on the left side with a '+' sign between them. - The formula (s) of the products are written on the right side with a '+' sign between them.
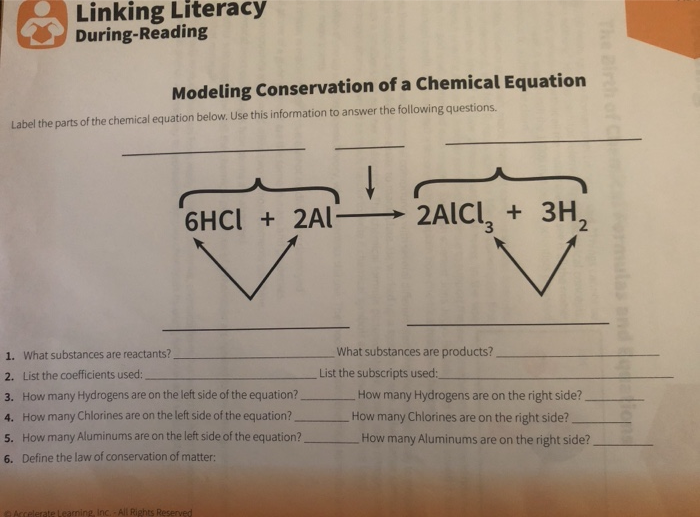
Al + HCl = AlCl3 + H2 - Chemical Equation Balancer WebLabel each compound (reactant or product) in the equation with a variable to represent the unknown coefficients. a Al + b HCl = c AlCl 3 + d H 2. Create a System of Equations. Create an equation for each element (Al, H, Cl) where each term represents the number of atoms of the element in each reactant or product.
How to Number or Label Equations in Microsoft Word - How-To Geek Click "New Label." In the New Label window, type your left parenthesis and hit "OK." If you want to select a different number format, click "Numbering," choose what you'd like to use, and click "OK." You'll see the starting parenthesis with the first number per the formatting that you selected. Type a space, and then your closing parenthesis.
Label the chemical Equation - Liveworksheets Label the chemical Equation worksheet Live worksheets > English Label the chemical Equation Label the parts of the chemical Equation ID: 1570824 Language: English School subject: Science Grade/level: 5 Age: 7-9 Main content: Chemical Equation Other contents: NA Add to my workbooks (0) Download file pdf Embed in my website or blog
What is a Chemical Equation? - Definition & Examples Chemical Equations = Reactions Because a chemical equation represents a chemical reaction, it's important to understand what a chemical reaction is. You observe chemical reactions...
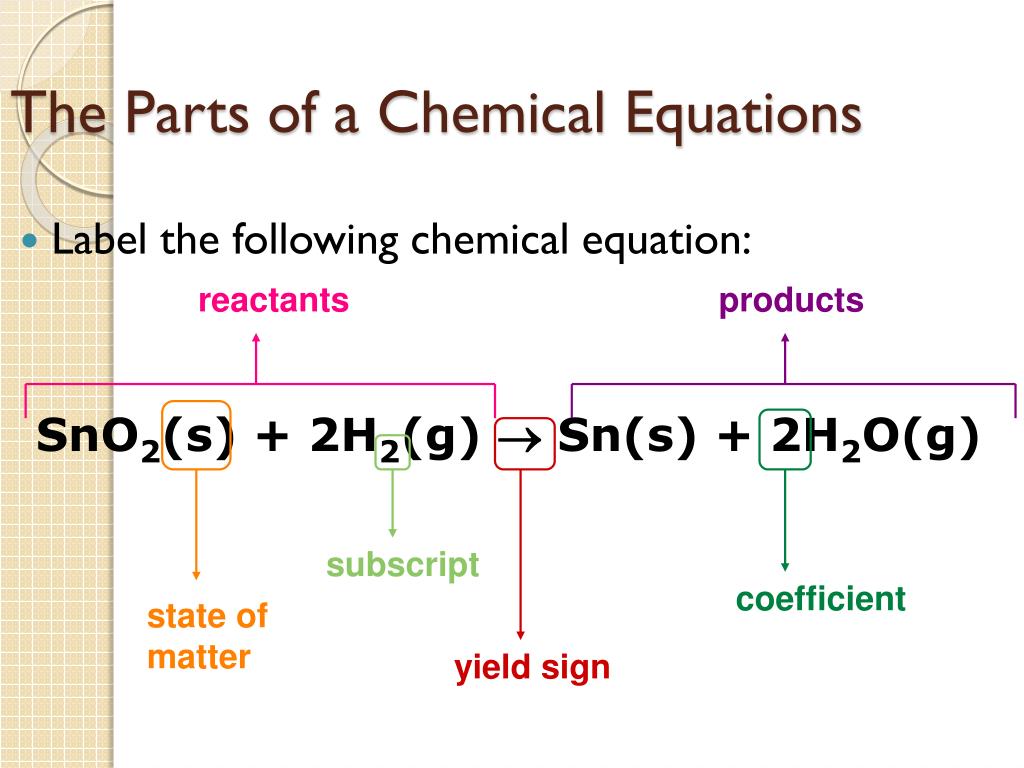
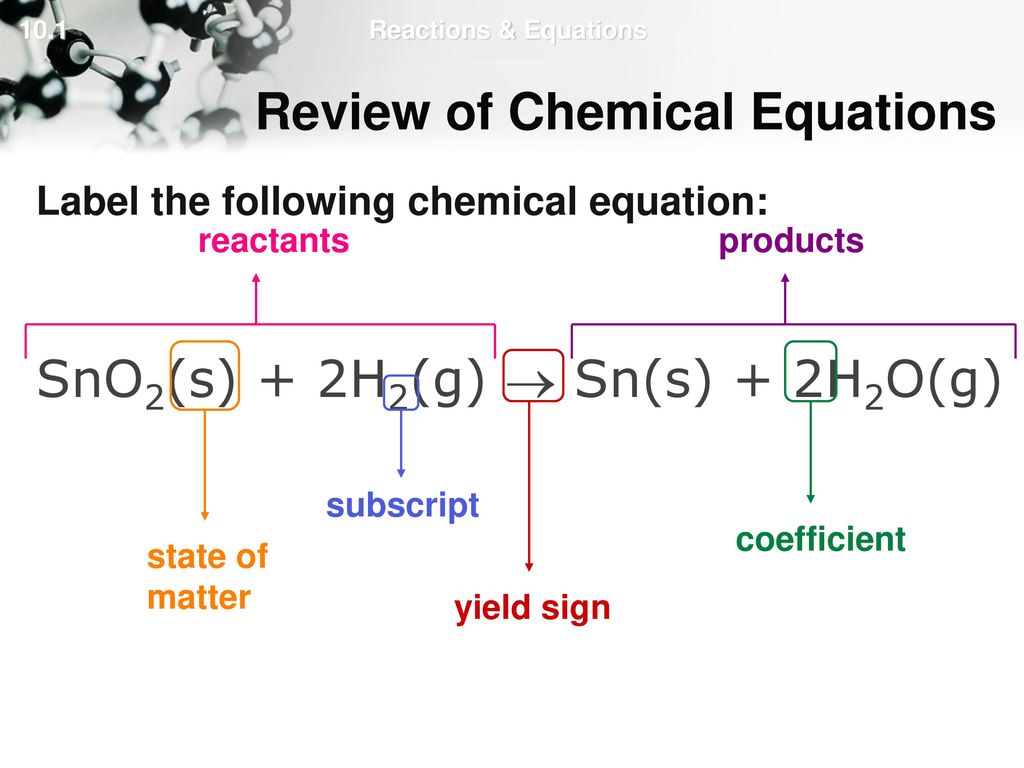
Label Chemical Equations - Labelled diagram Label Chemical Equations - Labelled diagram reactants, products, yield, coefficient, subscript, 2 molecules of water, 1 molecule of oxygen.
The Chemical Equation - Introductory Chemistry - 1st Canadian Edition This is an example of a chemical equation, which is a concise way of representing a chemical reaction. ... Many chemical equations also include phase labels for the substances: (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for aqueous (i.e., dissolved in water). Special conditions, such as temperature, may also be listed above the arrow.
How to Write a Chemical Equation (with Pictures) - wikiHow There are a few rules to keep in mind that help you identify the charges: [9] All group 1 elements at +1. All group 2 elements are +2. Transition elements will have Roman numerals in parentheses to indicate their charge. Silver is 1+, zinc is 2+, and aluminum is 3+. Group 17 elements are 1-. Group 16 elements are 2-. Group 15 elements are 3-.
Have your say WebThis site uses cookies to offer you a better browsing experience. Find out more on how we use cookies.
Symbols used in Chemical Equations Flashcards | Quizlet designates an aqueous solution; the substance is dissolved H₂O; placed after the chemical formula (s) or (c) means the substance is in a solid state (l) means the substance is in a liquid state (g) means the substance is in a gaseous state N.R. means no reaction will take place. chemical equation an expression representing a chemical reaction
KClO3 = KCl + O2 - Chemical Equation Balancer WebLabel each compound (reactant or product) in the equation with a variable to represent the unknown coefficients. a KClO 3 = b KCl + c O 2. Create a System of Equations. Create an equation for each element (K, Cl, O) where each term represents the number of atoms of the element in each reactant or product. K: 1 a = 1 b + 0c Cl: 1 a = 1 b + 0c O ...
Factor-Label Method in Chemistry: Definition, Examples & Practice ... The Factor-Label Method. Believe it or not, one simple method can be used to accomplish many of the basic calculations in chemistry. The method does not involve years of calculus courses or other ...
7.3: Chemical Equations - Chemistry LibreTexts Writing Chemical Equations. When sulfur dioxide is added to oxygen, sulfur trioxide is produced. Sulfur dioxide and oxygen, SO 2 + O 2, are reactants and sulfur trioxide, SO 3, is the product. 2SO 2(g) + O 2(g) ⏟ Reactants → 2SO 3(g) ⏟ Products. In chemical reactions, the reactants are found before the symbol " → " and the products are ...













Post a Comment for "38 label a chemical equation"