38 usda label approval form
Non-Hormone Treated Cattle Program | Agricultural Marketing Service All farms, ranches, and feedlots approved under these programs and that raise beef destined for shipment to the European Union (EU) as non-hormone treated cattle must be listed on the FSIS PartnerShare website. There must be verification that there are effective controls at the slaughter establishment. The EU export requirements are listed on ... Logo | USDA The USDA logo was created and approved for use in 1995. It is the official and sole identifying mark for the Department, its mission areas, and all agency programs. The logo is the single, most visible asset of our organization. ... Use the Signature Iso-Bar on all multimedia content, interfaces, applications (apps), labeling, and packaging.
askFSIS Public Q&As: FSIS Labeling Records - USDA The recordkeeping requirements for labeling records are found in 9 CFR 320.1 (b) (10) and 381.175 (b) (6). If you have any questions about the information in this Knowledge Article or any other questions about this topic, you can submit them to the FSIS Policy Experts at askFSIS by clicking on this link and filling out the web form to submit ...
Usda label approval form
FDA Label Search The drug labels and other drug-specific information on this Web site represent the most recent drug listing information companies have submitted to the Food and Drug Administration (FDA). (See 21 CFR part 207.) The drug labeling and other information has been reformatted to make it easier to read but its content has neither been altered nor ... USDA USDA Innovations. Each year, we develop new varieties of fruits, vegetables, and other products that provide consumers with improved convenience, longer shelf life, better nutrition, new flavors, and sometimes even a whole new idea that no one has brought to consumers before. Read the Story. Become a USDA Foods Vendor | Agricultural Marketing Service At any time during the process, questions may be directed to: Andrea Lang. New Vendor/Small Business Coordinator. USDA, AMS Commodity Procurement Staff. NewVendor@usda.gov. 202-720-4237. Livestock, Poultry, Fish, Dairy, Grain & Oilseed, International Packaged Commodities. Diana Dau David.
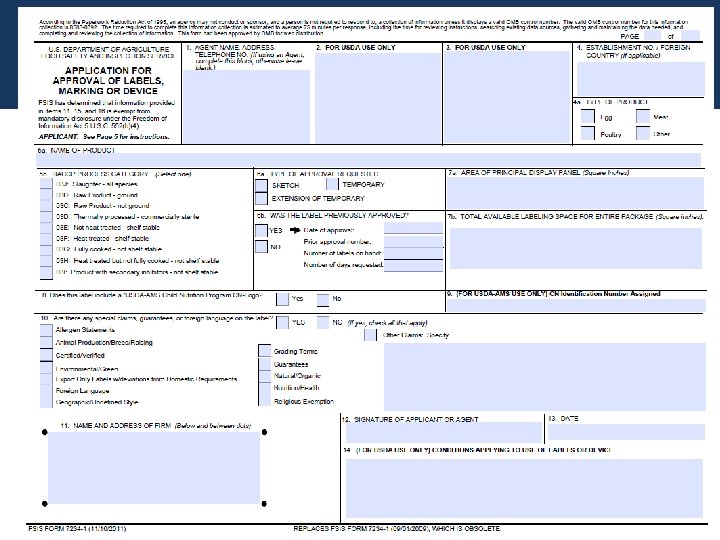
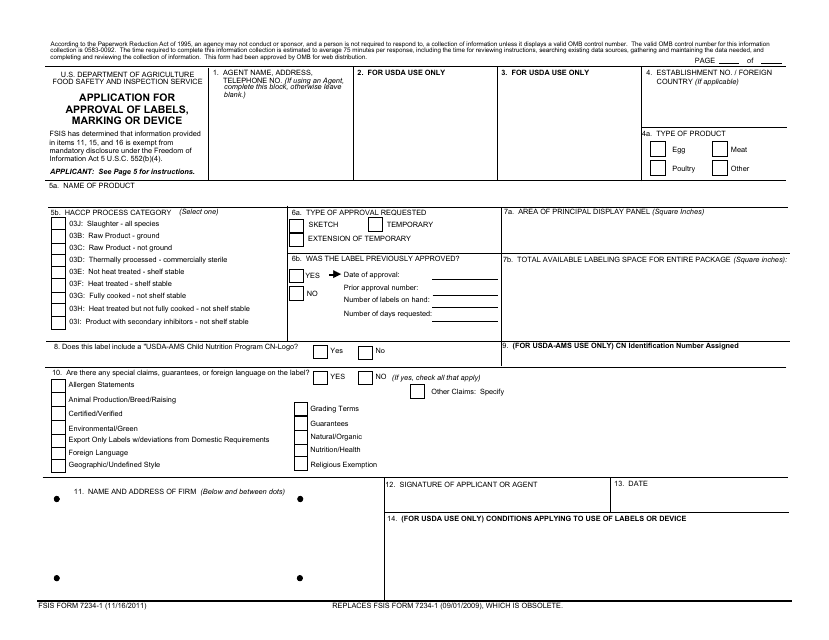
Usda label approval form. PDF FSIS 7234-1 Application for Approval of Labels, Marking or Device FSIS 7234-1 Application for Approval of Labels, Marking or Device According to the Paperwork Reduction Act of 1995, an agency may not conduct or sponsor, and a person is not required to respond to, a collection of information unless it displays a valid OMB control number. The valid OMB control number for this information collection is 0583-0092. Module 3 - USDA Labeling & Label Approval Process.pptx Label Approval Process and Generically Approved Labels USDA Label Pre-Approval • Prior label approval is a statutory requirement: • (d) Sales under false or misleading name, other marking or labeling or in containers of misleading form or size; trade names, and other marking, labeling, and containers approved by Secretary. PDF TRANSMITTAL OF LABELING OR OUTLINES - aphis.usda.gov Use separate forms for Outlines and labeling. 1. NAME AND FULL MAILING ADDRESS OF SUBMITTER Enter the establishment name and complete mailing address (street, city, state, ZIP) of the submitter. The processed form will be returned to official mailing address on file for the establishment. 2. DATE OF RELATED PRIOR CORRESPONDENCE Prime Label Consultants EZ Form software is a great tool for preparing for label approvals and managing them. The Deluxe version also provides assistance with Canadian regulations. Richard Young Labeling Compliance Manager, Quality AdvancePierre Foods Customized On-Site Training Tyson Foods wanted to ensure that their entire label development team was on the same page.
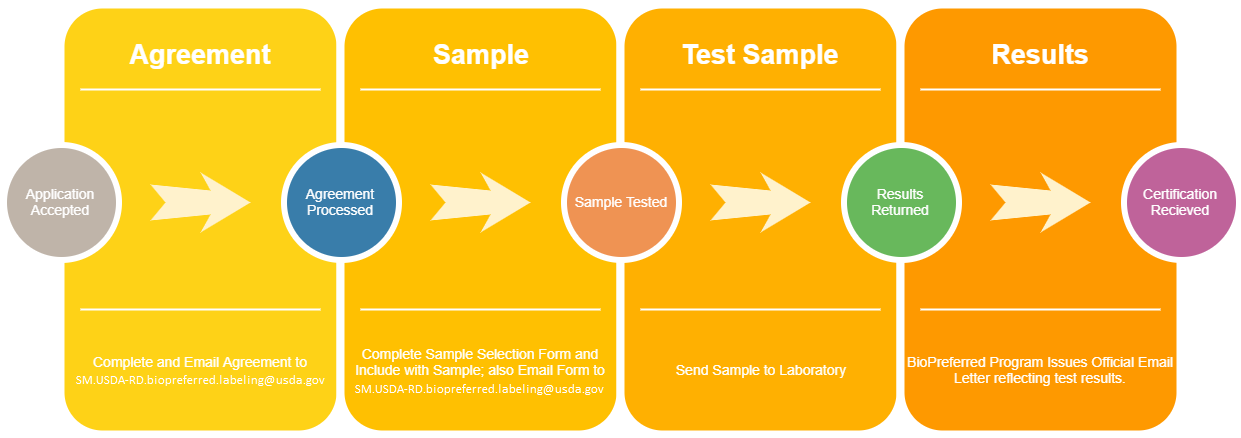
PDF USDA Services Order Form - Prime Label USDA Services Order Form USDA Services Order Form Prime Label prescreens labels and delivers them to the USDA within the specified time frame selected. Once at the USDA, label review time is variable and return dates cannot be guaranteed. Labeling and Label Approval | Food Safety and Inspection Service Labeling and Label Approval FSIS develops and provides labeling guidance, policies and inspection methods and administers programs to protect consumers from misbranded and economically adulterated meat, poultry, and egg products which ensure that all labels are truthful and not misleading. PDF Step by Step Instructions for Filling Out Federal and State Special ... labeling division. Fill out FSIS FORM 7234-1if your processor operates under USDA or TA inspection. Before filling out the application form, check with your processor to find out if they prefer to submit the label claim application to the federal labeling division or if they want you as the farmer to submit the application - both are acceptable. Prior Label Approval System: Expansion of Generic Label Approval specifically, under this proposal the following labels would no longer need to be submitted to fsis for approval: (1) labels on products for export that deviate from fsis requirements; (2) labels that list ingredients in the ingredients statement as being certified "organic" ( e.g., organic garlic) under the agricultural marketing service (ams) …
FSIS Labeling Overview and Generic Label Approval | Food Safety and ... This guidance also provides additional instruction on required labeling features, new generic labeling regulations, sample labels, label submission, and labeling records. It applies to food manufacturers and retailers and the actions they may take to comply with the label requirements applicable to meat and poultry. It relates to 9 CFR 412. What is required on a food label? - USDA A meat and poultry label is required to contain 8 features. These are: the product name, inspection legend and est. number, handling statement, net weight statement, ingredients statement, address line, nutrition facts, and safe handling instructions. These requirements are found in the Code of Federal Regulations (9CFR 317.2/381 Subpart N). Label Submission and Approval System (LSAS) | Food Safety and ... Mar 08, 2022 · Guidance document for submitting an FSIS Enrollment Request for the Label Submission and Approval System (LSAS) USDA eAuthentication System "eAuth" is the secure system that allows web-based access to USDA applications and services. This USDA site tells you how to obtain a Level 2 USDA eAuthentication account. Register for an eAuth Account USDA Foods Processing Templates and Forms End Product Data Schedule (EPDS) 1: Basic schedule for USDA Foods 100% Yield and Guaranteed Return is an excel file that can be used for both substitutable and non-substitutable USDA food end products. All end products to be processed must be submitted on an EPDS and approved by the applicable government agency.
PDF FSIS Compliance Guidance for Label Approval - Food Safety and ... The label application should indicate that the special claim will be added to an entire product line and should specify the product line, or, in the case of only certain labels, the application should list the product labels to which the approval would apply. Some examples include: •An establishment produces 50 different frozen meals.
AD Forms | National Finance Center - USDA The forms on this Web site are interactive fillable electronic forms - they can be downloaded and completed electronically. ... USDA Electronic Forms TSP Forms ASO Only Forms Pay Period Calendars Tax Information Taxes ... Recommendation & Approval of Awards: PDF, 761KB: AD-343: Payroll Action Request: PDF, 33KB: AD-349: Employee Address: PDF, 20KB:
USDA/USDC Authorized Labels and Manufacturers CN Label Manufacturers Report (608.23 KB) CN Label Verification Report (11.62 MB) CN numbers that appear on the valid list apply to the CN logo and crediting statement only. It is the manufacturer's responsibility to ensure that the product label meets all over federal labeling requirements. CN Label Verification Report (8/10/2022)
PDF Shell Egg Label Approval - Agricultural Marketing Service Approval notices, approved labels, or letters concerning printed labels that bear a USDA approval number will be distributed from the National Office to the label/consumer container manufacturer or packing plant, as applicable. When requested, copies will be sent to the distributor or general office. F. Reassignment of Approved Labels
Forms and Guidelines | USDA Form 6500-46 - Forest Service Authorization for In-Service Expenditures (PDF, 26.6 KB) GPO 907 Non-Compliance/Change Report (PDF, 33.9 KB) GPO 952 Desktop-Disk Information (PDF, 204 KB) GPO 1815 Notice of Quality Defects (PDF, 211 KB) GPO 3868 Notification of Intent To Publish (PDF, 115 KB) USDA Style Guide.
CN Labeling Program | Food and Nutrition Service - USDA The USDA, Child Nutrition (CN) Labeling Program provides food manufacturers the option to include a standardized food crediting statement on their product label. Labels must be authorized by USDA, FNS prior to use and manufacturers must have quality control procedures and inspection oversight that meet the FNS requirements.
USDA APHIS | Shipping Requirements for Importing Plant and Plant ... The United States Department of Agriculture (USDA), Animal and Plant Health Inspection Service (APHIS) is using electronic barcoded shipping labels for Plant Protection and Quarantine (PPQ) imports that require shipping labels. ... You may request the PPQ Form 508 (Green/Yellow labels) ... must be approved by USDA and/or CBP. Labeling Specifics
PDF United States Department of Agriculture Instructions: Please ... - Usda USDA-APHIS-PPQ . Permit Services : 4700 River Road, Unit 133 . ... PPQ Form 585 . OCT 2011 . Warning: Any alteration, forgery, or unauthorized use of this document is subject to civil penalties of up to $250,000 (7 U.S.C. 7734(b)) or punishable by ... Raw softwood lumber, without bark, must be consigned to an approved facility operating under a ...
FSIS Compliance Guideline for Label Approval | Food Safety ... Aug 24, 2021 · This guidance document provides official establishments information about the types of labels that must be submitted to LPDS for approval. Included are specific examples of special statements and claims that must be submitted to LPDS for approval. This guideline relates to FSIS labeling regulations in 9 CFR 412.
USDA APHIS | APHIS Electronic Forms Library APHIS Electronic Forms Library. Last Modified: Jul 27, 2020. Print. A current APHIS Forms Catalog lists all available APHIS and program forms and where the forms are stocked. The FGIS Forms Catalog, FGIS Obsolete Forms and the PSP Forms Catalog also are available. Contact Information.
Forms | Agricultural Marketing Service DA 228 - Request for Applicant Number Form Dairy Grading and Inspection Request Form DA155 - Application to Use Official ID DA156 - Request to Display Official ID DA157 - Request to validate a prior meat and poultry equipment acceptance DA162 - Equipment Review Request (Dairy) Research and Promotion Forms
USDA APHIS | APHIS Forms Form Number . Fillable . Description. Revision Date, Other Info. Format . APHIS 1. Yes. Request and Authorization for Occasional or Irregular Unscheduled Overtime. 03/1983. PDF. APHIS 12. Yes. Change Management Request Form. 03/2006. PDF. APHIS 14-R. Yes. FTS 2001 Calling Card Tracking Sheet Generic Cards Without Names. 10/2004. PDF. APHIS 16-R ...
Become a USDA Foods Vendor | Agricultural Marketing Service At any time during the process, questions may be directed to: Andrea Lang. New Vendor/Small Business Coordinator. USDA, AMS Commodity Procurement Staff. NewVendor@usda.gov. 202-720-4237. Livestock, Poultry, Fish, Dairy, Grain & Oilseed, International Packaged Commodities. Diana Dau David.
USDA USDA Innovations. Each year, we develop new varieties of fruits, vegetables, and other products that provide consumers with improved convenience, longer shelf life, better nutrition, new flavors, and sometimes even a whole new idea that no one has brought to consumers before. Read the Story.
FDA Label Search The drug labels and other drug-specific information on this Web site represent the most recent drug listing information companies have submitted to the Food and Drug Administration (FDA). (See 21 CFR part 207.) The drug labeling and other information has been reformatted to make it easier to read but its content has neither been altered nor ...



















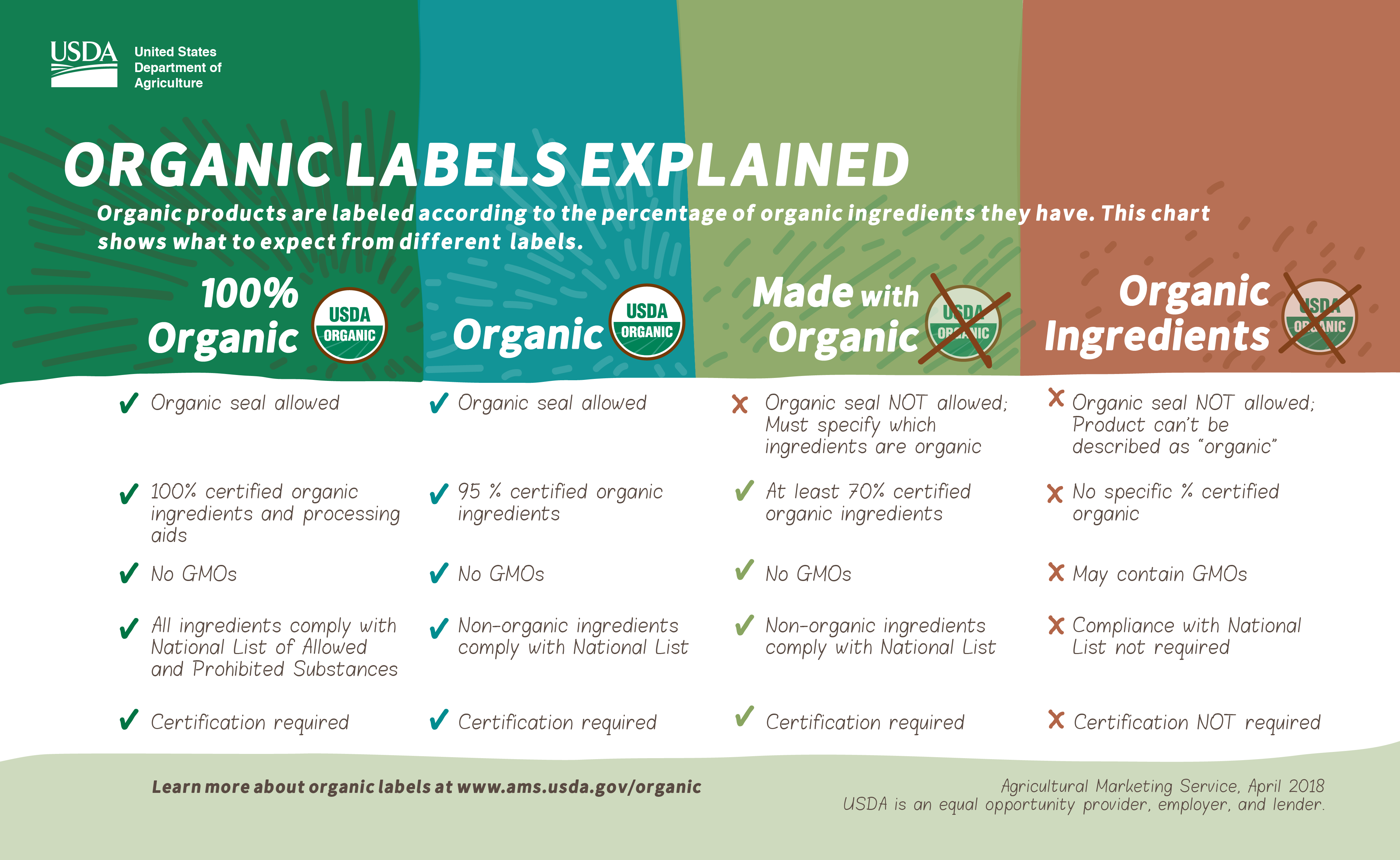
Post a Comment for "38 usda label approval form"