43 draw a ph scale and label it
pH Scale Flashcards | Quizlet pH Scale a scale used to define the levels of hydrogen (H) or hydroxide (OH) ions in a solution. Ranges from 0 (acidic) to 14 (basic/alkaline), with 7 being neutral. Hydrogen Ion an ion of hydrogen (H) created by the breaking up of a water molecule during the mixing of a solution. (Negatively charged) Hydroxide Ion Draw neat and labeled diagram of pH scale? - Toppr Ask The neat and labeled diagram of pH scale is as shown. The range of pH is from 0 to 14. pH 7 corresponds to neutral pH. pH less than 7 corresponds to acidic pH. pH more than 7 corresponds to alkaline pH. Was this answer helpful? 0 0
pH Scale: Acids, bases, pH and buffers (article) | Khan Academy The pH scale is often said to range from 0 to 14, and most solutions do fall within this range, although it's possible to get a pH below 0 or above 14. Anything below 7.0 is acidic, and anything above 7.0 is alkaline, or basic. Image modified from " Water: Figure 7 ," by OpenStax College, Biology, CC BY 4.0. Modification of work by Edward Stevens.
Draw a ph scale and label it
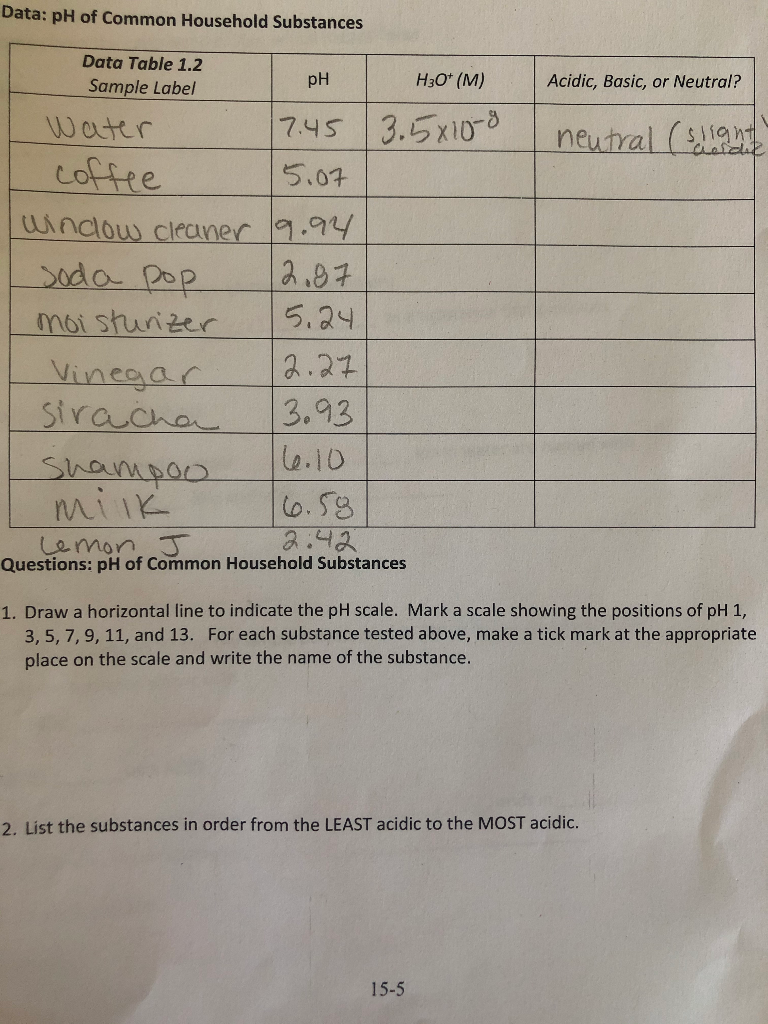
The pH Scale - Science Buddies To be more precise, pH is the negative logarithm of the hydrogen ion concentration: pH = −log [H+] The square brackets around the H + automatically mean "concentration" to a chemist. What the equation means is just what we said before: for each 1-unit change in pH, the hydrogen ion concentration changes ten-fold. Solved 1. Draw the pH scale from 0 to 14. 2. Indicate where - Chegg 1. Draw the pH scale from 0 to 14. 2. Indicate where on the scale it is acidic and basic and neutral. 3. At pH 3 and pH 12 value indicate the hydrogen ion concentration (For example at pH 7 hydrogen ion concentration is 0.0000001 M.) 4. On the scale show how hydrogen ion concentration changes (increases or decreases) from neutral to acidic and ... Solved: Draw the pH scale below, and indicate the following ... - Chegg 5LAA Draw the pH scale below, and indicate the following values: 0, 7 (neutral), and 14. Now label the scale with the names and indicate the location of the pH values for each substance you tested. Step-by-step solution This problem hasn't been solved yet! Ask an expert Back to top Corresponding textbook
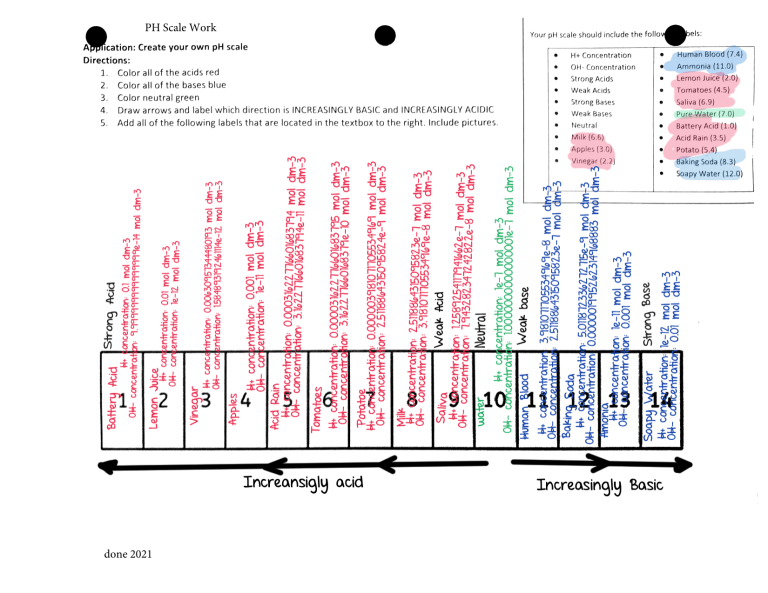
Draw a ph scale and label it. PPT Acids, Bases, and the pH scale - Mesa Public Schools The pH Scale A scale developed in order to determine how acidic or basic a substance is. Values on the scale range from 0-14. Indicator A substance that changes color in the presence of an acid or base. Ex. Cabbage Juice, litmus paper The pH Scale… Title: Acids, Bases, and the pH scale Author: User Last modified by ... pH Scale: Basics - pH | Acids | Bases - PhET Interactive Simulations Sample Learning Goals. Determine if a solution is acidic, basic or neutral. Place acids or bases in order of relative acidity or basicity. Relate liquid color to pH. Predict how solution volume or dilution with water will affect the pH of acids or bases. How do you draw the pH scale? - Answers The pH scale is often indicated as a vertical bar graph with scaled numbers from 0 to 14 (top to bottom). The lower numbers at the top are the more acidic pH, while the higher numbers near the... PDF pH Scale Activity - birdvilleschools.net On the construction paper, NEATLY draw a pH scale. 2. Scale the line from 0 to 14 with a mark for each number. 3. Cut out words & paste the labels in correct areas of pH scale. Weak Acid Strong Acid Strong Base Weak Base Neutral . 4. Color & Label the pH on the picture. Cut out & paste in the
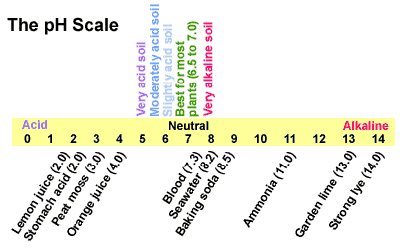
The pH scale with some common examples The pH scale, with examples of common solutions and their pH values. Download/View For commercial use please contact us 12 draw the ph scale using numbers and label acid Draw the pH scale using numbers and label acid, neutral, and base. (Using numbers 0-14) Acid AcidAcid Acid Acid AcidNeutral Base Base Base 10Base 11 Base 12 Base 13Base 141 23 4 5 67 8 9 Acid 1 Acid 2 Acid 3 Acid 4 Acid 5 Acid 6 Neutral 7 Base 8 Base 9 Base 10 Base 11 Base 12 Base 13 Base 14 13. What has more hydrogen ions, acids or bases? Base PH Scale in Simple Terms - YouTube What is the pH scale? The letters pH stand for the potential of hydrogen and is a measure of the concentration of hydrogen ions in a water-based substance?pH... 3. Draw a pH scale and label water, hydrochloric acid, and sodium ... 3. Draw a pH scale and label water, hydrochloric acid, and sodium hydroxide in their general areas on the scale. - 1161555
pH, pOH, and the pH scale (article) | Khan Academy The scale: Acidic, basic, and neutral solutions. Converting to is a convenient way to gauge the relative acidity or basicity of a solution. The scale allows us to easily rank different substances by their value. The scale is a negative logarithmic scale. pH Scale | U.S. Geological Survey - USGS.gov Each number represents a 10-fold change in the acidity/basicness of the water. Water with a pH of five is ten times more acidic than water having a pH of six. As this diagram shows, pH ranges from 0 to 14, with 7 being neutral. pHs less than 7 are acidic while pHs greater than 7 are alkaline (basic). Learn more about pH Sources/Usage Draw a pH scale and label water, hydrochloric acid, and sodi | Quizlet Find step-by-step Biology solutions and your answer to the following textbook question: Draw a pH scale and label water, hydrochloric acid, and sodium hydroxide in their general areas on the scale.. The pH scale - Acids, bases and salts - (CCEA) - BBC Bitesize The pH scale measures a solution's acidity or alkalinity. The range for the pH scale is 0 (strong acid) to 14 (strong alkali). pH 0 - 2: strong acid pH 3 - 6: weak acid pH 7: neutral pH 8 - 11:...
pH Scale - PhET pH Scale - PhET
Acids, Alkalis, and the pH Scale - Compound Interest On a simple level, the pH scale can be thought of as a ranking of the amount of hydrogen ions in a solution: the more hydrogen ions, the lower the pH number. The 'p' in pH, to chemists at least, stands for the mathematical operation '-log 10 '. pH, then, is simply equal to -log 10 [H + ], where [H + ] is the hydrogen ion concentration in a particular solution.
Label The Ph Scale Diagram / Science Worksheets Label Color The Ph ... The neat and labeled diagram of ph scale is as shown. Label The Ph Scale Diagram / Science Worksheets Label Color The Ph Scale By Science Workshop. a diagram a b diagram b c diagram c d diagram d. Find out what negative ph means. Ph less than 7 corresponds to acidic . The range of ph values. Draw neat and labelled diagram of ph scale.
pH Scale - Acids and Bases A pH of 7 is neutral on the scale, greater than 7 is a base and less than 7 is an acid. Strong acids are mostly ranged at a pH of 0-2, strong bases have a range at a pH of 12-14. Colour Indicators Colour Indicators are used to determine how acidic, basic or neutral the solution is.
pH Scale | U.S. Geological Survey - USGS.gov Objects that are not very acidic are called basic. The scale has values ranging from zero (the most acidic) to 14 (the most basic). As you can see from the pH scale above, pure water has a pH value of 7. This value is considered neutral—neither acidic or basic. Normal, clean rain has a pH value of between 5.0 and 5.5, which is slightly acidic.
pH and Dissociation of Water - University of Texas at Dallas If we want to understand the chemical state of a water, we must measure its pH as well as concentrations of dissolved species. Figure 4.3: Dissociation reaction of H O. Figure 4.4: The pH scale, where high pH indicates high concentrations of protons (H ions), and a high potential for proton donation. Next: Chemical Reactions and Temperature Up ...
The pH scale - BBC Bitesize The pH scale is a number scale from 0 to 14. It tells us how acidic or alkaline an aqueous solution is. The pH scale is used to classify as acidic, alkaline or neutral. Neutral solutions are...
pH Chemistry (Acids & Bases) - Definition, Calculating pH Value, Videos ... A pH scale is a tool for measuring acids and bases. The scale ranges from 0-14: Litmus paper is an indicator used to tell if a substance is an acid or a base. The colour of the paper matches up with the numbers on the pH scale to indicate what kind of substance is being tested. For example, Vinegar is an acid and measures 2.4 on the pH scale.
Solved: Draw the pH scale below, and indicate the following ... - Chegg 5LAA Draw the pH scale below, and indicate the following values: 0, 7 (neutral), and 14. Now label the scale with the names and indicate the location of the pH values for each substance you tested. Step-by-step solution This problem hasn't been solved yet! Ask an expert Back to top Corresponding textbook
Solved 1. Draw the pH scale from 0 to 14. 2. Indicate where - Chegg 1. Draw the pH scale from 0 to 14. 2. Indicate where on the scale it is acidic and basic and neutral. 3. At pH 3 and pH 12 value indicate the hydrogen ion concentration (For example at pH 7 hydrogen ion concentration is 0.0000001 M.) 4. On the scale show how hydrogen ion concentration changes (increases or decreases) from neutral to acidic and ...
The pH Scale - Science Buddies To be more precise, pH is the negative logarithm of the hydrogen ion concentration: pH = −log [H+] The square brackets around the H + automatically mean "concentration" to a chemist. What the equation means is just what we said before: for each 1-unit change in pH, the hydrogen ion concentration changes ten-fold.































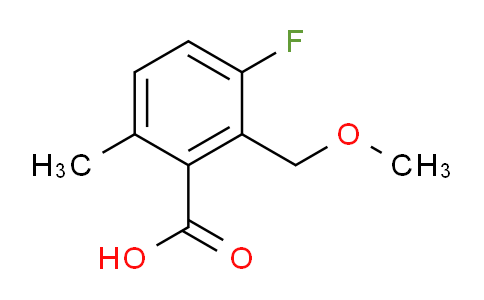
Post a Comment for "43 draw a ph scale and label it"